Functional Degree:
Assistant Professor/Penata/III.c
NIK: 156120103
NIDN: 0523058802
Place and date of birth:
Cilacap, 23 Mei 1988
Home address:
Jabung Gedhe No. 11
Pandowoharjo, Sleman
Kab. Sleman Di. Yogyakarta
Email:
[email protected]
Education background:
S.Si, Chemistry Department, Gadjah Mada University (2011)
M.Sc, Chemistry Department, Gadjah Mada University (2014)
PhD in Engineering, Environmental and Renewable Energy Systems, Gifu University (2021)
Teaching Courses:
Physical Chemistry II, Colloidal and Surface Chemistry
Math for Chemistry, Chemical Bond
Quantum Chemistry, Computational Chemistry
Physical Chemistry I
Nano Materials, Catalyst Chemistry
Practical Physical Chemistry
Expertise:
Physical Chemistry, Computational Chemistry and Materials
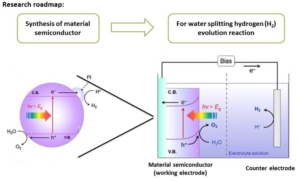
Molten Salt Synthesis of Nitride Semiconductor by Using Solid Nitrogen Source and Photochemical Activity (for Water Splitting and Solar Cell)
The main energy sources of the world are non-renewable and are faced with constantly increasing demand; therefore, these sources are not expected to last long. The world’s major energy sources are mainly the fossil fuels which contribute tremendously to the global warming problem we are facing today. The aforementioned problems related to the above energy sources make the international community focus attention on the alternative sources of energy, especially solar energy which seems highly promising. Solar energy is emitted from the sun primarily as electromagnetic radiation in the ultraviolet to infrared and radio spectral regions (0.2 to 3 m). The sun has a reasonable constant life time with a projected constant radiative energy output of over 10 billion years1. One of the device that uses sun as its energy source is the solar cell. A solar cell performs two major functions: photo generation of charge carriers in a light absorbing material and separation of the charge carriers to a conductive contact that will transmit the electricity. Solar cells are electronic devices used for the direct conversion of solar energy to electricity, using the photovoltaic (PV) effect2.
Related to the performance of the energy-conversion devices, nanomaterials have been extensively studied recently due to their specific properties which can enhance the performance of the device. The nanostructured materials have potential application in numerous fields which includes electronic devices, optoelectronics, optics, tribology, biotechnology, human medicine and others3,4. Nanostructured materials contain structures with dimensions in the nanometer length scale which includes polycrystalline materials with nanometer sized crystallites, materials with surface protrusions spatially separated by few nanometers granular or porous materials with grain sizes in the nanometer range or nanometer sized metallic clusters embedded in a dielectric matrix. The motivation for using nanostructured materials emerges from their specific physical and chemical properties. Enhancing the regular crystalline structure using nanocrystalline materials can increase the absorbance of all incident solar spectra in the form of thin films or multilayered solar cells. This increase requires an electrolyte to transfer the charge from the photo-acceptor to the electrodes in dye-based and polymer solar cells, whereas in nanoparticle based cells, the particles should be sufficiently close to one another to transfer the charge directly5,6,7.
[1] C.P. Poole Jr. and F.J. Owens, Introduction to Nanotechnology (Wiley, 2003).
[2] C.Wadia, Y. Wu, S. Gul, S. Volkman, J.Guo and A.P. Alivisatos, Chem. Mater. 2009, 21, 2568.
[3] B. Bhushan, Springer Handbook of Nanotechnology (Springer, 2004).
[4] T.Bradford, Solar Revolution (The MIT Press, 2006).
[5] P.V. Kamat, G.C. Schatz, J. Phys. Chem. C 2009, 113, 15437.
[6] U. Sahaym, N.M. Grant, J. Mater. Sci. 2008, 43, 5395.
[7] P.V. Kamat, J. Phys. Chem. Lett. 2011, 2, 242.










